HKUST Researchers Demonstrate Near-Non-Invasive In Vivo Imaging in Mouse Cortex at an Unprecedented Depth
A research team from the Hong Kong University of Science and Technology (HKUST) has demonstrated for the first time in vivo imaging of fine neuronal structures in mouse cortex through the intact skull at an unprecedented depth of 750µm below pia, making high-resolution microscopy in cortex near non-invasive and measurably facilitating the study of the living brain.
The direct and non-invasive visualization of neurons, glia, and microvasculature in the brain in vivo is critical for enhancing our understanding of how the brain functions. Over recent decades, great effort has been focused on developing novel techniques for in vivo imaging of the intact brain. However, none of the prevalent technologies, including ultrasound imaging (sonography), positron emission tomography (PET), and magnetic resonance imaging (MRI), provides sufficient spatial resolution to visualize biological structures at the subcellular level.
While optical microscopy such as three-photon microscopy (3PM) can provide structural and functional information in living specimens at high spatiotemporal resolution, optical aberration and scattering occur as light travels through and interacts with inhomogeneous biological tissues, fundamentally limiting the performance of optical microscopy in both resolution and depth.
Although adaptive optics (AO) is a possible solution to correct for the aberration and restore the resolution of in vivo optical microscopy, it is not without shortcomings: the guide star signal for the conventional wavefront sensing fades away quickly when imaging depth increases.
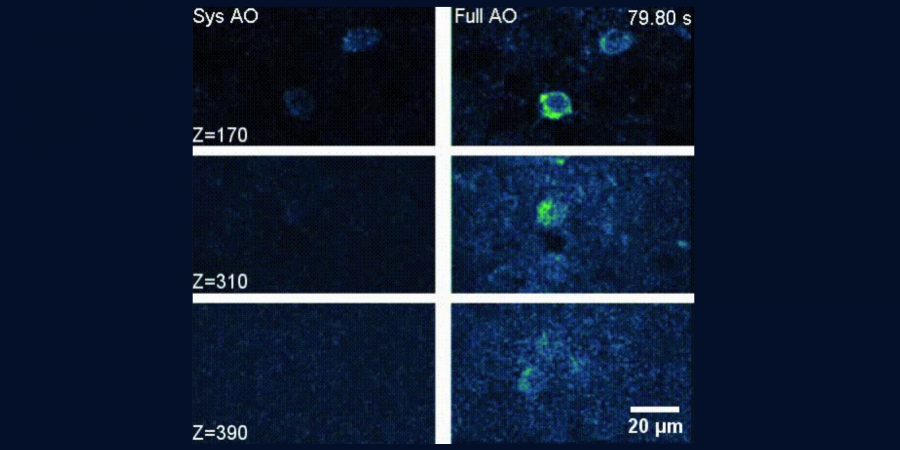
Now, co-led by Prof. QU Jianan, Professor in Department of Electronic and Computer Engineering, and Prof. Nancy IP, Chair Professor in Division of Life Science, a HKUST research team developed a microscope that combines 3PM with two forms of AO, demonstrating fast measurements and the correction of both low-order and high-order aberrations in tissue at great depth.
The technology makes use of two AO techniques: direct focus sensing with phase-sensitive detection and conjugate adaptive optics (CAO) with remote focusing. The guide star signal is coded and then decoded in the aberration measurement to achieve AO correction of aberrations. These enable the accurate measurement of the aberrant electric-field point-spread function of a laser in tissue and the fast correction of the aberration over a large imaging volume in the brain.
The team validated the imaging performance of the AO-3PM system using a wavelength of 1300nm, imaging through intact skull both in vivo and on in vitro preparations. The results showed that AO-3PM achieved high spatial resolution with a drastically improved fluorescence signal over a large depth, and high-resolution in vivo structural and functional imaging of mouse cortices through the intact skull up to 750µm below the pia mater.
Further, by using a pupil AO-3PM, the team achieved high-resolution imaging of subcortical structures up to 1.1mm below the pia mater within the intact brain. Taking advantage of the tight focus provided by their unique AO technique, the team went on to demonstrate the capability of AO-3PM to guide precise laser microsurgery and investigate post-operative microglial dynamics in the cortex through the intact skull.
“It is absolutely fun to exploit the marriage of electronics and optics to bring a new tool for experimental biology,” Prof. Qu said. “Overall, our results demonstrate that AO-3PM technology holds great potential to advance in vivo imaging techniques and facilitate study of living brain.”
“It is truly remarkable what this state-of-the-art AO-3PM system can achieve,” Prof. Ip explained. “The high performance and unparalleled accuracy of this advanced deep-brain imaging technology will substantially widen our understanding of living brain with optimal physiological representation.”
The research findings were recently published in Nature Biotechnology.
(This news was originally published on EurekAlert on June 13, 2022.)