HKUST Researchers Discovered Mutation Route That Helps Find New Therapeutic Lead for Deadly Brain Cancer Patients
A research team consisting of scientists from the Hong Kong University of Science and Technology (HKUST) and Beijing Tiantan Hospital have uncovered the mutational mechanism of how a rare and deadly brain cancer –secondary glioblastoma (sGBM) – progresses from its less lethal type. The groundbreaking finding has provided a therapeutic lead which may develop into a new kind of treatment for chemo-resistant patients.
Among the 200 new cases of aggressive brain tumor recorded in Hong Kong each year by the Hospital Authority, about a quarter are lower-grade glioma (LGG) tumors. Starting off at nerve cells around the spine and brain, LGG is the early form of sGBM – one of the deadliest brain cancers known today. While sGBM can be treated with surgery or an oral chemotherapy drug called temozolomide (TMZ) – most of these malign tumors would mutate and return again with mortality rate reaching almost 100 per cent. Until now, the genomic features and evolution mechanism of the progression from LGG to sGBM remain elusive.
In their latest study, the team led by Prof. WANG Jiguang, Assistant Professor at HKUST’s Division of Life Science and Department of Chemical and Biological Engineering, identified METex14 mutations at MET oncogene as a major culprit behind this aggressive progression. The team analyzed and integrated the genomic data of 188 sGBM patients – including newly collected samples from Chinese and South Korean patients, with a specially designed computational model and found that about 14% of the sampled sGBM patients displayed mutations in this gene.
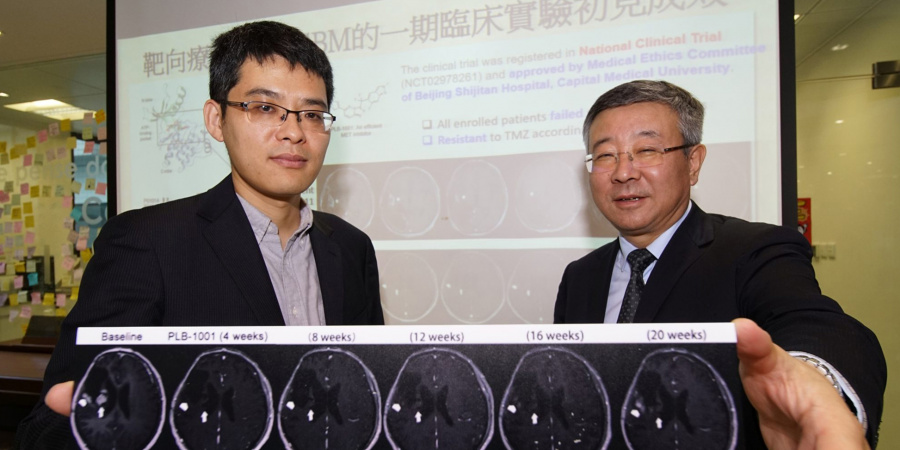
Taking reference from this discovery, HKUST’s collaborators – Prof. JIANG Tao and his team from Beijing Neurosurgical Institute and Beijing Tiantan Hospital, identified a drug molecule named PLB-1001 that is able to penetrate the blood-brain barrier – a physiological structure that sees separation of blood circulation and extracellular fluid in the central nervous system – and reach the tumors in the brain. The molecule shows remarkable potency in selectively targeting sGBM tumors and those displaying further gene mutations.
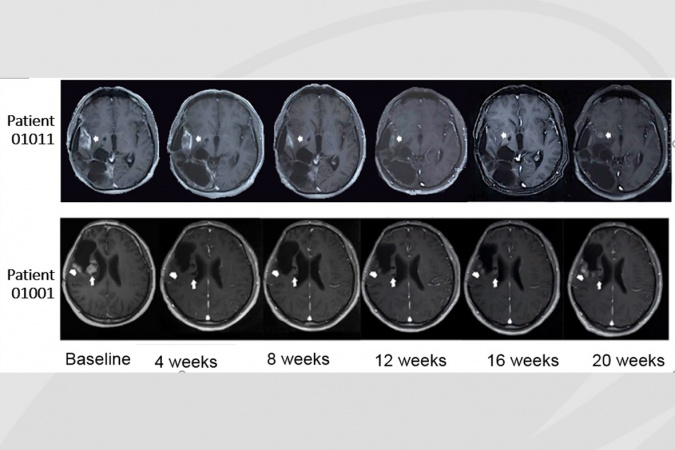
The clinical trial of PLB-1001 on 18 recurrent patients at late cancer stage returned with a partial positive response in two patients. After being prescribed a daily dosage ranging from 50 to 300 mg at Beijing Tiantan Hospital, the two patients experienced significant tumor shrinkage, with relieved symptoms and little side effects, lasting for more than 12 weeks.
“More studies on PLB-1001 are needed to see if it can be used in conjunction with other drugs in achieving more persistent results,” said Prof Wang from HKUST. “But the outcome of this clinical trial is significant in a sense that it furthers the knowledge about sGBM treatment. Developing computational models on cancer evolution helps predict cancer cells’ future behavior and prioritize treatment options, while precision cancer medicine promises to tailor treatments according to personal cancer mutations, although the dynamic changes during cancer evolution add to its complication. sGBM tumor is high on our target list as it is one of the toughest tumors to treat.”
The findings were published in the top scientific journal Cell on November 29, 2018. This paper is a follow-up work of Prof. Wang’s three recent publications in Nature Genetics (Wang et al. 2016; Lee et al. 2017; Lee et al. 2018).
(This news was originally published by the HKUST Public Affairs Office here.)